A highly fluorinated lithium iron phosphate with interpenetrating lattices: electrochemistry and ionic conductivity†
Abstract
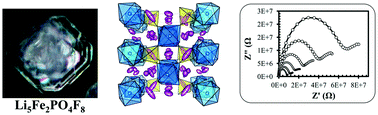
Li5Fe2PO4F8, a new member of the family of alkali transition metal fluorophosphates, has been synthesized and characterized using single-crystal X-ray diffraction, 57Fe Mössbauer spectroscopy and magnetic susceptibility measurements. The existence of an infinite {–[PO4(FeF4)2]–}∞ tetrahedral network in an inter-penetrated diamond lattice, along with the presence of seven unique Li sites, presents interesting structural features of this structure-type for energy storage applications. The initial results of (de)lithiation reveal that a relatively low fraction of theoretical capacity may be utilized reversibly (0.2 Li+ ion per formula unit), possibly due to the lack of available free volume for Li+ insertion. The high Li content and the existence of large channels in all 3-dimensions of space also offer opportunities to study this material as a candidate for solid-state electrolytes. The results from electro-impedance measurements reveal the reasonable activation energy of Li diffusion (0.70 eV), which is also supported by theoretical calculations.


 Please wait while we load your content...
Please wait while we load your content...