Reactivity of N-heterocyclic carbene–pyridine palladacyclopentadiene complexes toward halogen addition. The unpredictable course of the reaction†
Abstract
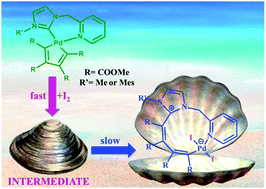
As an extension of a previously published work we have reacted some palladacyclopentadiene complexes stabilized by bidentate N-heterocyclic carbene–pyridine or monodentate N-heterocyclic carbene–pyridine and isocyanide ligands with the halogens I2 and Br2. All the bidentate and monodentate complexes react with halogens to give at first the expected σ-coordinated butadienyl fragment. However, two of the less hindered NHC carbene–pyridine bidentate butadienyl iodo derivatives undergo a further rearrangement and novel Pd(II) complexes characterized by a ten term coordinative ring were isolated and characterized. In the most favorable case we were able to carry out the kinetics of rearrangement and measure its reaction rate. Moreover, we have surmised a plausible mechanism on the basis of a dedicated computational approach and in one case the surprising structure characterized by the ten term coordinative ring was resolved by X-ray diffraction.


 Please wait while we load your content...
Please wait while we load your content...