Synthesis and evaluation of biological properties of ferrocenyl–podophyllotoxin conjugates†
Abstract
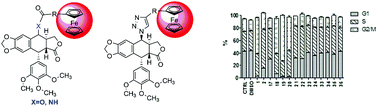
Three types, esters, amides and 1,2,3-triazoles, of ferrocenyl–podophyllotoxin conjugates were synthesised, and their anticancer activity was evaluated. We observed that the most potent ferrocenyl derivatives were esters. Esters 15, 16 and 17 acted in a similar way to podophyllotoxin, i.e. reduced the number of G1 phase cells and induced G2/M blockage, while esters 14 and 18 and amide 19 blocked cells in S phase in a similar manner to etoposide.


 Please wait while we load your content...
Please wait while we load your content...