Thermal stability of mesoscopic compounds of cetyltrimethylammonium and Keggin metatungstates†
Abstract
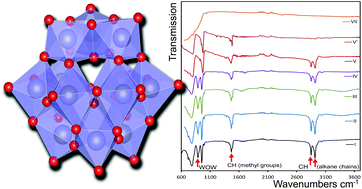
A hybrid surfactant/polyoxometalate compound was synthesized by combining isopolytungstate anions with the cationic surfactant cetyltrimethylammonium bromide (CTA-Br) to produce a hierarchical compound that we identify as (CTA)7[H2W12O40]Cl·2H2O. At room temperature the compound consisted of hexagonally ordered sheets of Keggin ions, with an intervening gallery containing alkyl-chains of the organic cations. The synthesis was highly dependent on solution pH, reaction time and the order in which the reactants were added. We examined the effect of temperature on the stability of (CTA)7[H2W12O40]Cl·2H2O using thermal gravimetric analysis, differential scanning calorimetry, FT-IR spectroscopy and in situ synchrotron X-ray diffraction, and found a step-wise conversion to monoclinic WOxvia a series of intermediates. Heating under nitrogen atmospheres accelerated transition events by ∼100 °C when compared to heating in air. During heating, the interplanar gallery at first expanded in a series of steps starting at 90 °C as the CTA+ amphiphiles changed orientation, before collapsing rapidly at 240 °C, a temperature coinciding with the removal of about 40% of the organic material. Between 240 and 320 °C, the material consisted of fragments of the Keggin ion cores, arranged in 2D hexagonally-packed sheets. At ∼330 °C, the Keggin ions were completely destroyed and replaced by bulk W17O47 which, upon further heating, transformed to bulk WO2 or WO3 depending on the environment.


 Please wait while we load your content...
Please wait while we load your content...