Electrochemical and structural investigation of the interactions between naphthalene diimides and metal cations†
Abstract
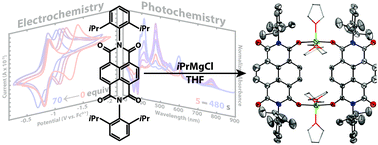
The effects of Li+ and Mg2+ on the electrochemical behaviour and photoreduction of N,N′-bis(2,6-diisopropylphenyl)naphthalene diimide (Dipp2NDI) have been investigated using cyclic voltammetry and UV-vis spectroscopy. Strong cation–anion interactions between Li+ or Mg2+ and [Dipp2NDI]2− result in solvent-dependent anodic shifts of the NDI˙−/2− redox couple. In organic solvents of moderate dielectric constant and donor ability, the two, one-electron redox processes typically observed for Dipp2NDI merge into a single, two-electron process. This strong metal cation effect has been leveraged for the photoreduction of Dipp2NDI to [Dipp2NDI]2−via a disproportionation process. Chemical reduction of Dipp2NDI with iPrMgCl has allowed the isolation of Mg2+ complexes of [Dipp2NDI]2−. Single crystal X-ray diffraction was used to determine the structure of a dimeric complex, [(Dipp2NDI)Mg2(THF)3]2, that features strong coordination of Mg2+ by the oxygen atoms of the reduced NDI.


 Please wait while we load your content...
Please wait while we load your content...