Control over cyclisation sequences of 1,1′-bifunctional octamethylferrocenes to ferrocenophanes†
Abstract
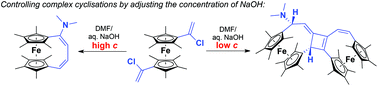
This paper describes the facile synthesis of a number of electron rich octamethyl[1.4]ferrocenophanes with unsaturated handles from 1,1′-bis(1-chlorovinyl)octamethylferrocene. Treatment of this reactive compound with sodium hydroxide in DMF initiates a series of reactions resulting in the formation of four different ferrocenophanes. The most complex of these products arises from a cascade of cyclisations giving an unusual, unsymmetrical bis-ferrocenophane with a central fused cyclobutene. Control over the reaction outcome is achieved by manipulating the concentration of NaOH. Mechanisms are proposed, and supported by DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...