Facile activation of alkynes with a boraguanidinato-stabilized germylene: a combined experimental and theoretical study†
Abstract
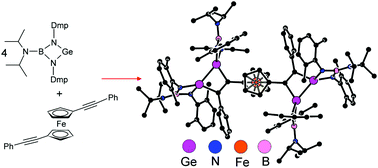
A boraguanidinato-stabilized germylene, [(i-Pr)2NB(N-2,6-Me2C6H3)2]Ge, reacts with alkynes RC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR selectively in a 2 : 1 molar ratio to afford 3,4-R,R′-1,2-digermacyclobut-3-enes 1a–e as the products of formal [2 + 2 + 2] cyclization [R/R′ = Me/Me (1a), Ph/Ph (1b), Ph/H (1c), t-Bu/H (1d) and Cy/H (1e)]. Ferrocenyl-substituted alkynes react similarly, yielding the corresponding ferrocenylated 3,4-R,R′-1,2-digermacyclobut-3-enes 2a–d [where R/R′ = Fc/H (2a), Fc/Me (2b), Fc/Ph (2c), and Fc/Fc (2d); Fc = ferrocenyl]. By contrast, only one of the triple bonds available in conjugated diynes RC
CR selectively in a 2 : 1 molar ratio to afford 3,4-R,R′-1,2-digermacyclobut-3-enes 1a–e as the products of formal [2 + 2 + 2] cyclization [R/R′ = Me/Me (1a), Ph/Ph (1b), Ph/H (1c), t-Bu/H (1d) and Cy/H (1e)]. Ferrocenyl-substituted alkynes react similarly, yielding the corresponding ferrocenylated 3,4-R,R′-1,2-digermacyclobut-3-enes 2a–d [where R/R′ = Fc/H (2a), Fc/Me (2b), Fc/Ph (2c), and Fc/Fc (2d); Fc = ferrocenyl]. By contrast, only one of the triple bonds available in conjugated diynes RC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC
CC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR is activated with the germylene, while the second one remains intact even in the presence of an excess of the germylene. The exclusive formation of 3,4-R,(C
CR is activated with the germylene, while the second one remains intact even in the presence of an excess of the germylene. The exclusive formation of 3,4-R,(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)-1,2-digermacyclobut-3-enes 3a–c [R = Ph (3a), t-Bu(3b), and Fc (3c)] was ascribed to a steric repulsion around the second triple bond. On the other hand, the reaction of the germylene with more flexible dialkyne fc(C
CR)-1,2-digermacyclobut-3-enes 3a–c [R = Ph (3a), t-Bu(3b), and Fc (3c)] was ascribed to a steric repulsion around the second triple bond. On the other hand, the reaction of the germylene with more flexible dialkyne fc(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)2 (fc = ferrocene-1,1′-diyl) proceeded in the expected manner, producing compound 4, where both triple bonds are transformed into 1,2-digermacyclobut-3-ene rings by reaction with four equivalents of the germylene. All compounds were characterized by multinuclear NMR spectroscopy, Raman and IR spectroscopy, and in the case of 1a–c, 2a, 2c, 3a, 3b and 4, also by single-crystal X-ray diffraction analysis. The ferrocenyl substituted compounds were studied by cyclic voltammetry (CV). Finally, the plausible reaction pathway was studied for a model reaction of [(i-Pr)2NB(N-2,6-Me2C6H3)2]Ge with MeC
CPh)2 (fc = ferrocene-1,1′-diyl) proceeded in the expected manner, producing compound 4, where both triple bonds are transformed into 1,2-digermacyclobut-3-ene rings by reaction with four equivalents of the germylene. All compounds were characterized by multinuclear NMR spectroscopy, Raman and IR spectroscopy, and in the case of 1a–c, 2a, 2c, 3a, 3b and 4, also by single-crystal X-ray diffraction analysis. The ferrocenyl substituted compounds were studied by cyclic voltammetry (CV). Finally, the plausible reaction pathway was studied for a model reaction of [(i-Pr)2NB(N-2,6-Me2C6H3)2]Ge with MeC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CMe using DFT computations.
CMe using DFT computations.

 Please wait while we load your content...
Please wait while we load your content...