Di- vs. tetra-substituted quinonediimines: a drastic effect on coordination chemistry†
Abstract
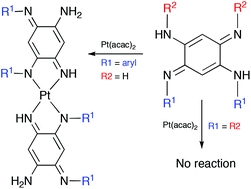
We describe the first isolation of N,N′-disubstituted benzoquinonediimines (QDIs) 2 which revealed drastically different properties compared to N,N′,N′′,N′′′-tetrasubstituted QDIs 1 due to the absence of N-substituents in the “upper part” of the molecule. Three major differences could be highlighted thanks to the present joint experimental and theoretical investigation: (1) the metalation with different metal precursors revealed a specific behaviour in coordination chemistry giving access to novel complexes with unprecedented stoichiometry and remarkable absorption properties. More specifically, a new monoplatinum complex that is able to absorb light from the UV up to the NIR region because of its unique delocalization pathway across the metal centre was obtained; (2) this new class of di-substituted QDIs showed an exceptionally broad pKa range (ΔpKa ∼ 8) that could be explained by the possible tuning of the basicity of each imine which is now attainable; (3) these quinones could be used as reagents in organic synthesis to form the previously unknown N,N′,N′′,N′′′-tetrasubstituted QDIs with tuneable substituents (aryl vs. alkyl), previously unknown, as a new ligand for coordination chemistry.


 Please wait while we load your content...
Please wait while we load your content...