4,4′-Di-tert-butyl-6-(1H-tetrazol-5-yl)-2,2′-bipyridine: modification of a highly selective N-donor ligand for the separation of trivalent actinides from lanthanides†
Abstract
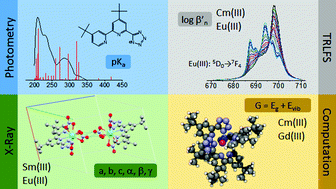
In the present work, the complexation and extraction behaviour of 4,4′di-tert-butyl-6-(1H-tetrazol-5-yl)-2,2′-bipyridine (HN4tbubipy) towards trivalent actinides (An(III)) and lanthanides (Ln(III)) is studied by spectroscopic methods, liquid–liquid extraction, and quantum chemical calculations. The ligand synthesis of HN4tbubipy as well as its application in coordination chemistry of the 4f elements is described. Reaction of HN4tbubipy with [Ln(NO3)3·6H2O] (Ln = Sm, Eu) results in [H2N4tbubipy]+[Ln(N4tbubipy)(NO3)3(H2O)]−. Both compounds have been characterized by single crystal X-ray diffraction. The solubility of the ligand in different organic solvents is determined, showing a high solubility in MeOH which decreases with the lipophilicity of the solvent. The pKa = 2.4 ± 0.2 of HN4tbubipy in EtOH (4.4 vol% H2O) is determined by absorption spectrophotometry. The complexation of Cm(III) and Eu(III) with HN4tbubipy is studied by time resolved laser fluorescence spectroscopy (TRLFS). For both metal ions the formation of the complexes [M(N4tbubipy)n]3−n with n = 2, 3 (M = Cm(III), Eu(III)) is observed. Slightly higher conditional stability constants for Eu(III) (log β′2(Eu(N4tbubipy)2+) = 8.9 ± 0.3, log β′3(Eu(N4tbubipy)3) = 12.7 ± 0.5), compared to Cm(III) (log β′2(Cm(N4tbubipy)2+) = 8.5 ± 0.4 and log β′3(Cm(N4tbubipy)3) = 12.4 ± 0.6) are determined. Thus, the ligand has no preference for the complexation of An(III) over Ln(III). Additionally, no significant extraction of Am(III) and Eu(III) is observed in liquid–liquid extraction experiments due to protonation of the ligand at the experimental conditions. The experimental studies are supported by quantum chemical calculations of the free ligand and the [M(N4tbubipy)3] complexes (M = Cm(III), Gd(III)). The results are in excellent agreement with the experimental data and provide a deeper understanding of the complexation properties of HN4tbubipy.


 Please wait while we load your content...
Please wait while we load your content...