Structure stability of HKUST-1 towards water and ethanol and their effect on its CO2 capture properties†
Abstract
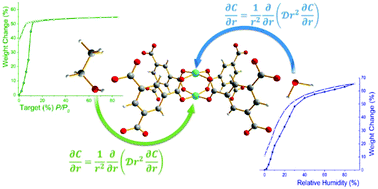
Water and ethanol stabilities of the crystal structure of the Cu-based metal–organic framework (MOF) HKUST-1 have been investigated. Vapour (water and ethanol) sorption isotherms and cyclability were measured by a dynamic strategy. The ethanol sorption capacity of HKUST-1 at 303 K remained unchanged contrasting water sorption (which decreased along with the sorption experiment time). Considering the binding energy of each sorbate with the open Cu(II) sites, obtained by the use of diffusion coefficients, we showed the superior crystal stability of the HKUST-1 framework towards ethanol. Finally, a small quantity of ethanol (pre-adsorbed) slightly enhanced CO2 capture without crystal structure degradation.


 Please wait while we load your content...
Please wait while we load your content...