Highly reducible π-extended copper corroles†
Abstract
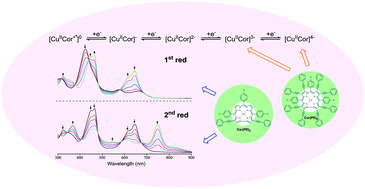
Di- and octa-phenylethynyl (PE) substituted π-extended copper corroles were synthesized and characterized as to their structural, electrochemical and spectroscopic properties. The addition of two or eight PE groups to the β-pyrrole positions of the corrole results in dramatic red shifts in the electronic absorption spectra and new reductions which are not seen for the parent compound lacking PE substituents. CuCor(PE)8 is reduced in four reversible one-electron transfer steps to give derivatives of [CuCor(PE)8]n− where n = 1, 2, 3 or 4. Variable temperature 1H NMR and EPR measurements were carried out and suggest that the octa- and di-PE substituted Cu-corroles can both be described as an antiferromagnetically coupled CuII corrole cation radical which is in equilibrium with a triplet state, possibly due to a lower singlet–triplet energy gap as compared to 1 and 2 at room temperature. The EPR spectra of one-electron oxidized and one electron reduced species exhibited the characteristics of Cu(II) corroles. The products generated in the first two reductions of each π-extended corrole were characterized by thin-layer spectroelectrochemistry, thus providing new insights into how UV-vis spectra of highly reduced corroles vary as a function of the number of PE groups and overall charge on the molecule. The singly reduced and singly oxidized copper corroles were also chemically generated in CH3CN and shown to have UV-visible spectra almost identical to the spectra obtained by electroreduction or electrooxidation in PhCN or THF containing 0.1 M tetrabutylammonium perchlorate.


 Please wait while we load your content...
Please wait while we load your content...