Carbene stabilized interconnected bis-germylene and its silicon analogue with small methyl substituents†
Abstract
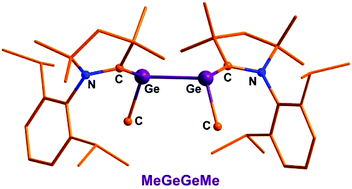
The cyclic alkyl(amino) carbene (cAAC) [:C{N-C6H3(2,6-IPr2)}(CMe2)2CH2] stabilized MeGeGeMe has been isolated in the molecular form with composition (cAAC)MeGe–GeMe(cAAC) (1) at room temperature. Compound 1 was synthesized from the reduction of MeGeCl3 using three equivalents of KC8 in the presence of one equivalent of cAAC. The corresponding silicon compound (cAAC)MeSi–SiMe(cAAC) (2) was also prepared. Compounds 1 and 2 are the first examples of REER compounds (E = Ge, Si) carrying the smallest organic group. Furthermore the structures of compounds 1 and 2 have been investigated by using theoretical methods. The theoretical analysis of the structure of 1 is in agreement with the formation as an unprecedented carbene stabilized bis-germylene whereas compound 2 can be equally described as carbene stabilized bis-silylene with coordinate bonds as with classical double bonds of a 2,3-disila-1,3-butadiene. The compounds were also characterized by X-ray crystallography.


 Please wait while we load your content...
Please wait while we load your content...