Controlling the oxidation of bis-tridentate cobalt(ii) complexes having bis(2-pyridylalkyl)amines: ligand vs. metal oxidation†
Abstract
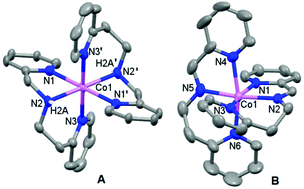
Two bis-tridentate chelated cobalt(II) complexes, which differ in the ligand structure by a methylene group, activate molecular oxygen (O2), and give different oxidation products. The O2 reaction of [CoII(pepma)2]2+ (1) with unsymmetrical 2-(2-pyridyl)-N-(2-pyridylmethyl)ethanamine (pepma) results in ligand oxidation, to the corresponding Co(II) imine complex [CoII(pepmi)2]2+ (2). Contrastingly, the Co(II) complex [CoII(bpma)2]2+ (3) of similar symmetrical bis(2-pyridylmethyl)amine (bpma), undergoes metal oxidation, yielding a cobalt(III) complex, [CoIII(bpma)2]2+ (4). The reversibility of the amine to imine conversion and the stability of the Co(II) imine complex (2) are investigated. Furthermore, the solution dynamics of Co(II) complexes are highlighted with the help of paramagnetic 1H-NMR spectroscopy.


 Please wait while we load your content...
Please wait while we load your content...