Copper induced spin state change of heme–Aβ associated with Alzheimer's disease†
Abstract
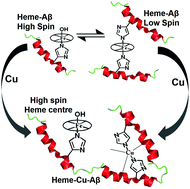
Heme binds Aβ to give a mixture of a mono-histidine bound high spin peroxidase type active site and a bis-histidine bound low spin cytochrome b type active site present in an equilibrium at physiological pH. Of these, the high spin mono-histidine bound complexes produce significant amounts of partially reduced oxygen species (PROS), catalyze the degradation of neurotransmitters and oxidize cytochrome c, with potentially detrimental effects. The presence of excess Aβ could lower these effects by creating a low spin bis-histidine cytochrome b type active site which exerts less oxidative stress by producing a much smaller amount of PROS. The presence of Cu(II) reverses this effect and can convert the benign low spin heme–Aβ complex to the detrimental high spin form, even in the presence of excess Aβ. Data suggest that the histidine needed to form the bis-histidine site in the low spin heme–Aβ complex is likely to be involved in the high affinity Cu binding site in the heme–Cu–Aβ complex.
- This article is part of the themed collection: Frontiers in Spectroscopic Techniques in Inorganic Chemistry


 Please wait while we load your content...
Please wait while we load your content...