Covalent hybrids based on Re(i) tricarbonyl complexes and polypyridine-functionalized polyoxometalate: synthesis, characterization and electronic properties†
Abstract
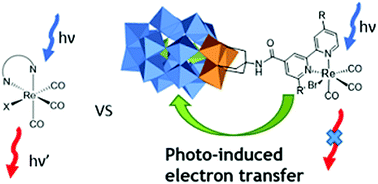
A series of [Re(CO)3Br(N^N)] (N^N = substituted 2,2′-bipyridine ligand) complexes based on polypyridine-functionalized Dawson polyoxometalate (1–3) has been synthesized. The new hybrids (4–6) were characterized by various analytical techniques, including absorption, vibrational and luminescence spectroscopies as well as electrochemistry. Both units, the polyoxometalate and the transition metal complex, retain their intrinsic properties. Their combination in the newly prepared hybrids results in improved photosensitization in the high-energy visible region. However, a complete quenching of the emission for the [Re(CO)3Br(N^N)] complexes is observed due to formation of a charge separated state, Re(II) – POM−, as shown by quenching experiments as well as theoretical modelling via DFT.


 Please wait while we load your content...
Please wait while we load your content...