A new set of Cd(ii)-coordination polymers with mixed ligands of dicarboxylate and pyridyl substituted diaminotriazine: selective sorption towards CO2 and cationic dyes†
Abstract
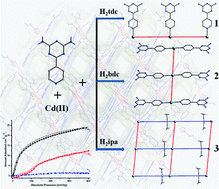
On the basis of a mixed ligand system of L(NH2)2 (6-(pyridin-4-yl)-1,3,5-triazine-2,4-diamine) and dicarboxylic acids, three new Cd(II) coordination polymers viz. {[Cd0.5(tdc)0.5(L(NH2)2)0.5(H2O)]·DMF·H2O}n (1), {[Cd0.5(bdc)0.5(L(NH2)2)(H2O)]·DMF·H2O}n (2), and {[Cd(ipa)(L(NH2)2)(DMF)]·H2O}n (3) (tdcH2 = thiophene-2,5-dicarboxylic acid, bdcH2 = benzene-1,4-dicarboxylic acid, ipaH2 = benzene-1,3-dicarboxylic acid) were synthesized under diverse reaction conditions and characterized by single crystal X-ray diffraction, PXRD, elemental analysis, IR spectroscopy and TGA. While 1 and 2 revealed 1D chain structures, 3 acquired a 2D square net structural arrangement. Gas adsorption measurements of the desolvated framework 3 showed a moderate uptake of CO2 under ambient conditions with good selectivity over N2 and CH4. The solid state luminescence properties were studied for all three coordination polymers. Moreover, a dye adsorption study on 3 exhibited selective adsorption towards a cationic dye.


 Please wait while we load your content...
Please wait while we load your content...