The ability of a zinc pyrrolidine complex to catalyze the synthesis of cyclic carbonates from carbon dioxide and epoxides: a mechanistic theoretical investigation†
Abstract
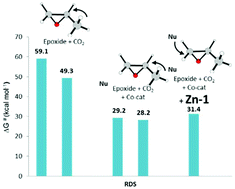
The reaction mechanism for the synthesis of cyclic carbonates from carbon dioxide and epoxides catalyzed by a zinc pyrrolidine complex has been elucidated using the density functional level of theory. The obtained potential energy surface shows that the recently proposed zinc complex is able to efficiently and selectively catalyze the formation of cyclic carbonate using carbon dioxide. In the proposed mechanism, the reaction occurs in two steps: in the first step, the epoxide cycle is activated by iodide nucleophile, whereas in the second step, carbon dioxide is inserted into the oxy-anion species to form the cyclic carbonate. The rate determining step is the epoxide opening process, which requires 31.6 kcal mol−1. The entire reaction results to be exergonic by 11.8 kcal mol−1. Comparison with the uncatalyzed process reveals that the presence of the co-catalyst and catalyst contribute not only to lower the activation energy but also to determine the regioselectivity.


 Please wait while we load your content...
Please wait while we load your content...