The underlying factors controlling the Pd-catalyzed site-selective alkenylation of aliphatic amines†
Abstract
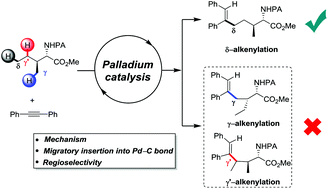
The Pd(II)-catalyzed site-selective δ-C(sp3)–H alkenylation in the presence of more accessible γ-C(sp3)–H bonds is investigated by DFT calculations. Migratory insertion is found to be both the rate-limiting and the selectivity-determining step. The origin of the unusual site-selectivity is originally attributed to the different steric repulsion between the alkyne and palladacycle; however, our theoretical results reveal that the inherent electronic effect instead of steric repulsion determines the site-selectivity. The proposal is further validated by model calculations involving the less sterically hindered 1,2-dimethyl acetylene and acetylene. In addition, a novel HCO3−-assisted N–H activation mechanism is reported, and the origin of the regioselectivity of an unsymmetrical alkyne is also studied.


 Please wait while we load your content...
Please wait while we load your content...