Palladium(ii) in liquid ammonia: an investigation of structural and dynamical properties by applying quantum mechanical charge field molecular dynamics (QMCF-MD)†
Abstract
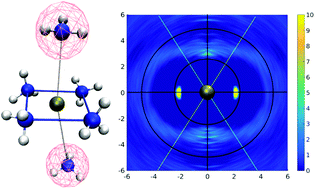
The structural and dynamical properties of Pd2+ in liquid ammonia have been investigated via quantum mechanical charge field molecular dynamics. Similar to the case of aqueous Pd2+, a six-fold coordination polyhedron in the form of a tetrahedrally elongated octahedron is observed with two ligands in axial positions forming an extended first shell. To highlight the difference in solvation between the aqueous and ammonia case a selection based on the angular-radial distribution with respect to the well-known square planar motif was applied also providing a detailed understanding of ligand exchange between the extended first and second shells. All structural properties resulting from this investigation compare well with the available solid-state data of various N-containing complexes. From the dynamical perspective, Pd2+ in liquid ammonia forms a more flexible complex with a higher rate of ligand exchange than that of its aqueous counterpart.


 Please wait while we load your content...
Please wait while we load your content...