Abstract
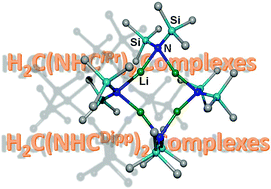
Lithiation of HN(SiMe3)2 in hexane and crystallization in the presence of 2,2,5,5-tetramethyltetrahydrofuran (Me4THF) allows the isolation of crystalline tetrameric [LiN(SiMe3)2]4 (1) with an average Li–N distance of 208.9 pm. Deprotonation of a methylene-bridged bis(imidazolium) diiodide with NaN(SiMe3)2 in tetrahydrofuran (THF) yields 1,1′-diisopropyl-3,3′-methylene-diimidazole-2,2′-diylidene (H2C(NHCiPr)2) and layering of this orange reaction mixture with pentane yield [{H2C(NHCiPr)2}{(thf)NaI}2]∞ (2). The reaction of this bis(imidazolium) diiodide with excess of KN(SiMe3)2 in THF leads to the formation of [{H2C(NHCiPr)2}KN(SiMe3)2]2 (3). Due to the fact that 2 and 3 are polymeric in the crystalline state, the bulkier 1,1′-bis(2,6-diisopropylphenyl)-3,3′-methylene-diimidazole-2,2′-diylidene (H2C(NHCDipp)2) has been used. Thus, addition of H2C(NHCDipp)2 with the bulky 2,6-diisopropylphenyl (Dipp) groups to AN(SiMe3)2 (A = Li, Na, K) in toluene yields mononuclear [{H2C(NHCDipp)2}LiN(SiMe3)2] (4) and dinuclear [{H2C(NHCDipp)2}AN(SiMe3)2]2 for sodium (5) and potassium (6). The rather short alkali metal–carbon bonds lie in the range of alkylmetal derivatives.


 Please wait while we load your content...
Please wait while we load your content...