Spectrally-resolved third-harmonic generation and the fundamental role of O–H⋯Cl hydrogen bonding in Oh, Td-cobalt(ii) tetraphenylmethane-based coordination polymers†
Abstract
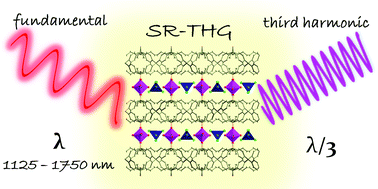
The reaction of a phosphonate-diester tetraphenylmethane-based tecton, tetrakis[4-(diethoxyphosphoryl)phenyl]methane, (L) with cobalt(II) chloride afforded a centrosymmetric coordination polymer (CP), [L·2Co(H2O)42+·2CoCl42−]n, 2-Cl, possessing simultaneously octahedral (Oh) and tetrahedral (Td) metal centers. This material served as a model compound for the demonstration of factors influencing the spectral dependence of one of the nonlinear optical (NLO) phenomena, the third-harmonic generation (THG). The spectrally-resolved THG (SR-THG) measurements in the range from 1125 to 1750 nm revealed that a maximum of THG response is obtained when the fundamental beam is around 1300 nm. The SR-THG study was combined with an analysis of the self-absorption effects of pumping and of third-harmonic radiation; based on these results, we put forward a hypothesis that the THG action spectrum is influenced more by the ability of the material to self-absorb the third harmonic rather than by the extent of self-absorption of the pumping radiation. Apart from investigations of NLO properties, we have explored coordination and particularly the supramolecular interactions that build up the 2-Cl CP. Despite the tetrahedral, spatial shape of the ligand L, CP 2-Cl has a two-dimensional net. The structure was found to be strongly supported by O–H⋯Cl hydrogen bonds, since each CoCl42− complex anion is an acceptor of eight of such interactions within a distorted square grid layer of cobalt(II) ions. While coordination and hydrogen-bonded nets are both featuring the sql topology when treated separately, the consideration of both of them as topological paths yields a trinodal 4,4,6-connected net, described by the point symbol (42·84)(45·6)2(46·66·83)2. SR-THG and structural studies of 2-Cl have been also supported by far- and mid-infrared spectroscopy, UV-Vis-NIR solid state absorption analysis, thermogravimetry and preliminary magnetic characterization.


 Please wait while we load your content...
Please wait while we load your content...