Optimising the relaxivities of Mn2+ complexes by targeting human serum albumin (HSA)†
Abstract
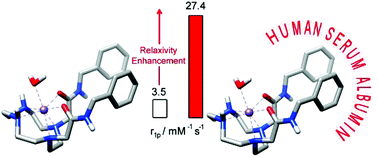
We report two novel macrocyclic ligands based on the 1,4-DO2AM platform (1,4-DO2AM = 2,2′-(1,4,7,10-tetraazacyclododecane-1,4-diyl)diacetamide) and containing two benzyl groups attached either to the nitrogen atoms of the macrocyclic unit (1,4-BzDO2AM) or to the amide pendant arms (1,4-DO2AMBz). The protonation constants of the ligands and the stability constants of their Mn2+ complexes were determined using pH potentiometry. The introduction of benzyl groups results in a slight decrease of the stability constants of the Mn2+ complexes and a slight increase of their acid-catalysed dissociation reactions. A detailed relaxometric characterisation of the complexes using nuclear magnetic dispersion relaxation (NMRD) and 17O NMR studies indicated that the increase in molecular weight associated with the presence of benzyl groups results in a remarkable increase of proton relaxivities r1p, which take values of 3.8, 3.5 and 2.5 mM−1 s−1 for [Mn(1,4-BzDO2AM)]2+, [Mn(1,4-DO2AMBz)]2+ and [Mn(1,4-DO2AM)]2+ (at 25 °C and 20 MHz). The [Mn(1,4-BzDO2AM)]2+ and [Mn(1,4-DO2AMBz)]2+ complexes form relatively strong adducts with Human Serum Albumin (HSA) with association constants of (3.9 ± 0.6) × 103 and (2.0 ± 0.3) × 103 M−1, respectively. The interaction with the protein slows down the rotational tumbling of the complex in solution, which results in adducts endowed with remarkably high proton relaxivities (r1pb = 18.5 ± 0.7 and 27.4 ± 1.4 mM−1 s−1 for [Mn(1,4-BzDO2AM)]2+ and [Mn(1,4-DO2AMBz)]2+, respectively).


 Please wait while we load your content...
Please wait while we load your content...