Spectroscopic and reactivity differences in metal complexes derived from sulfur containing Triphos homologs†
Abstract
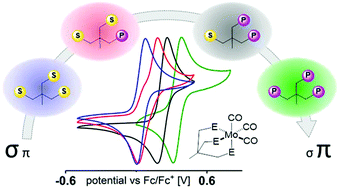
Herein, we report a simplified method for the synthesis of Triphos homologs H3CC(CH2X)n(CH2Y)3−n (X = SPh, Y = PPh2, n = 0–3). The multidentate compounds were tested for their potential to coordinate metals such as Ni, Fe, and Mo under the same experimental conditions. Cyclic voltammetry, spectroelectrochemical IR investigations as well as DFT calculations were used to examine the electronic alterations in a series of [{H3CC(CH2X)n(CH2Y)3−n}Mo(CO)3] complexes and to evaluate their potential to open coordination sites or to release CO upon oxidation or in the presence of different solvents. In addition, we demonstrate that the catalytic hydrosilylation of N,N-dimethylbenzamide to N,N-dimethylbenzylamine is influenced by the applied tripodal ligand. Our investigations show the high potential of such manipulations to selectively alter the dynamics of the binding properties of Triphos-metal complexes and their reactivity.
- This article is part of the themed collection: Frontiers in Spectroscopic Techniques in Inorganic Chemistry


 Please wait while we load your content...
Please wait while we load your content...