Liquid-phase oxidation of alkylaromatics to aromatic ketones with molecular oxygen over a Mn-based metal–organic framework†
Abstract
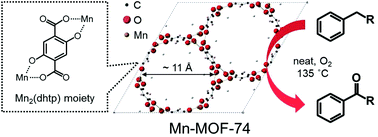
Liquid-phase oxidation of alkylaromatics with molecular O2 was examined using a microporous Mn-based metal–organic framework (Mn-MOF-74). Mn-MOF-74 consisting of trimeric Mn clusters and 2,5-dihydroxyterephthalate (dhtp) linkers exhibits superior catalytic activity with good ketone selectivity compared to conventional oxide-supported Mn catalysts without showing any lengthy induction period. Combined analyses by means of XRD, FE-SEM, N2 physisorption and Mn K-edge XAFS reveal that the superior catalytic performance is attributed to the inherently-formed Mn(III)2(dhtp) moieties embedded in the Mn-MOF-74 framework rather than structural factors associated with the MOF. The catalyst is reusable over multiple catalytic runs along with retaining its original catalytic activity due to the ability of the dhtp ligand to stabilize active Mn(III) atoms. Owing to high activity, reusability and nontoxicity, Mn-MOF-74 can offer a simple, inexpensive and efficient protocol for the oxidation of some important alkylaromatics, such as ethylbenzene and diphenylmethane to produce the corresponding aromatic ketones.


 Please wait while we load your content...
Please wait while we load your content...