Reaction mechanism of the metallohydrolase CpsB from Streptococcus pneumoniae, a promising target for novel antimicrobial agents†
Abstract
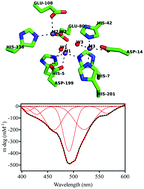
CpsB is a metal ion-dependent hydrolase involved in the biosynthesis of capsular polysaccharides in bacterial organisms. The enzyme has been proposed as a promising target for novel chemotherapeutics to combat antibiotic resistance. The crystal structure of CpsB indicated the presence of as many as three closely spaced metal ions, modelled as Mn2+, in the active site. While the preferred metal ion composition in vivo is obscure Mn2+ and Co2+ have been demonstrated to be most effective in reconstituting activity. Using isothermal titration calorimetry (ITC) we have demonstrated that, in contrast to the crystal structure, only two Mn2+ or Co2+ ions bind to a monomer of CpsB. This observation is in agreement with magnetic circular dichroism (MCD) and electron paramagnetic resonance (EPR) data that indicate the presence of two weakly ferromagnetically coupled Co2+ ions in the active site of catalytically active CpsB. While CpsB is known to be a phosphoesterase we have also been able to demonstrate that this enzyme is efficient in hydrolyzing the β-lactam substrate nitrocefin. Steady-state and stopped-flow kinetics measurements further indicated that phosphoesters and nitrocefin undergo catalysis in a conserved manner with a metal ion-bridging hydroxide acting as a nucleophile. Thus, the combined physicochemical studies demonstrate that CpsB is a novel member of the dinuclear metallohydrolase family.
- This article is part of the themed collection: Frontiers in Spectroscopic Techniques in Inorganic Chemistry


 Please wait while we load your content...
Please wait while we load your content...