Cyclometalated Ir(iii) complexes based on 2-(2,4-difluorophenyl)-pyridine and 2,2′-(2-phenyl-1H-imidazole-4,5-diyl)dipyridine: acid/base-induced structural transformation and luminescence switching, and photocatalytic activity for hydrogen evolution†
Abstract
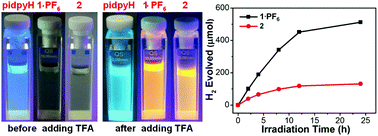
Based on ligands dfppyH and pidpyH, cyclometalated Ir(III) complexes [Ir(dfppy)2(pidpyH)](PF6) (1·PF6) and [Ir(dfppy)2(pidpy)] (2) have been synthesized. The crystal structures indicate that each {Ir(dfppy)2}+ unit is coordinated by a neutral ligand pidpyH in 1·PF6, while by a pidpy− anion in 2. The packing structure of 1·PF6 only exhibits electrostatic interactions and van der Waals interactions among [Ir(dfppy)2(pidpyH)]+ cations and PF6− ions. In contrast, the neighboring molecules in 2 are linked into a supramolecular chain structure through aromatic stacking interactions between two dfppy− ligands. In solution, 1·PF6 and 2 show acid/base-induced structural transformation due to the protonation/deprotonation of their pyridyl groups and/or imidazole units, which can be confirmed by their 1H NMR spectra. At room temperature, compounds 1·PF6, 2 and pidpyH in CH2Cl2 reveal TFA-induced luminescence switching behaviors, from a non-luminescence state to a luminescence state with an emission at 582 nm for both 1·PF6 and 2, and emission switching from 392 nm to 502 nm for pidpyH. These switching behaviors are associated with the protonation of pyridyl groups and/or imidazole units in 1·PF6, 2 and pidpyH. Moreover, compounds 1·PF6 and 2 were used as photosensitizers (PS) for reduction of water to hydrogen under the same experimental conditions. It was found that the amount of evolved hydrogen and the PS turnover number are 512 μmol and 102 for 1·PF6, and 131 μmol and 26 for 2, respectively. Thus, compound 1·PF6 has better photocatalytic activity than 2. In this paper, we discuss the modulation of luminescence and photocatalytic activities of 1·PF6 and 2 by varying the coordination mode and/or protonation extent of pidpyH/pidpy− ligands.


 Please wait while we load your content...
Please wait while we load your content...