Azaborines: synthesis and use in the generation of stabilized boron-substituted carbocations†
Abstract
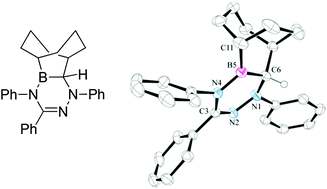
A formal N-heterocyclic carbene insertion into the B–H bond of 9-BBN followed by a ring expansion reaction is reported. NHC-9-BBN adducts were reacted in one or two steps to give the corresponding di- or triazaborines. Hydride abstraction of selected species with [Ph3C]+ is facile, giving rise to 6π-aromatic cations with Lewis acidity comparable to Lewis acids commonly employed in frustrated Lewis pairs.
- This article is part of the themed collection: CSC100: Celebrating Canadian Chemistry


 Please wait while we load your content...
Please wait while we load your content...