Stable AuIII complexes with four N-heterocyclic carbene groups can be prepared in high yield directly from KAuCl4†
Abstract
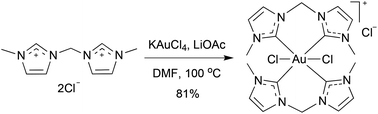
Gold(III) N-heterocyclic carbene (NHC) complexes of form [Au(NHC)4Cl2]Cl were synthesized by reaction of KAuCl4 with bis- and tetrakis(imidazolium) salts in the presence of a mild base. Treatment of these complexes with KPF6 afforded four-coordinate AuIII complexes of form [Au(NHC)4](PF6)3. X-Ray crystallography showed the [AuIII(NHC)4]3+ cations in the hexafluorophosphate salts to have a square planar Au(NHC)4 moiety [Au⋯CNHC 2.024(4)–2.082(7) Å]. In the [AuIII(NHC)4Cl2]+ cations in the chloride salts, coordination about Au was tetragonally-distorted octahedral, the axial Au–Cl bonds being substantially longer [Au⋯Cl 3.148(2)–3.693(1) Å] than the equatorial Au–CNHC bonds [Au⋯C 2.024(4)–2.082(7) Å]. NMR and conductance studies suggested that the structures of the complexes seen in the solid state persisted in DMSO solution, except in one case where a chlorido ligand dissociated from [AuIII(NHC)4Cl2]+ to form [AuIII(NHC)4Cl]2+. The AuIII(NHC)4 unit was surprisingly robust. An AuIII complex was found to undergo H/D exchange reactions in D2O solution at 100 °C with no signs of decomposition detectable by 1H NMR spectroscopy. 1H NMR studies showed that various complexes containing AuIII(NHC)4 moieties underwent little or no decomposition when heated at 120 °C in DMSO-d6 for extended periods.


 Please wait while we load your content...
Please wait while we load your content...