The mechanism change by switching the reactants from water to hydroxyl ions for electrocatalytic water oxidation: a case study of copper oxide microspheres†
Abstract
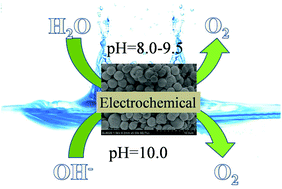
Developing noble metal-free water oxidation catalysts is essential for many energy conversion/storage processes (e.g., water splitting). Herein, we report a facile synthesis of CuO microspheres composed of ultrathin, single-crystal-like nanosheets via a simple solution method. The as-obtained CuO microspheres can serve as an active and stable water oxidation catalyst under electrochemical reaction conditions, owing to their unique structural features. In electrochemical water oxidation, this catalyst affords a current density of 10 mA cm−2 (a value related to practical relevance) at an overpotential of ∼0.48 V. Pure CuO was reported as a water oxidation catalyst (WOC) from near-neutral conditions to alkalescent conditions. Electrochemistry values agree with the Nernstian behavior, suggesting ne−/nH+ transfer prior to a chemical rate-determining step. Our results suggest that the delicate nanostructure can offer unique advantages for developing efficient water oxidation catalysts.


 Please wait while we load your content...
Please wait while we load your content...