Low temperature synthesis study of metal–organic framework CPO-27: investigating metal, solvent and base effects down to −78 °C†
Abstract
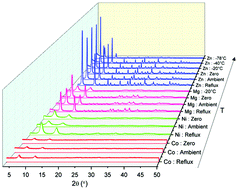
CPO-27-M (M = Co, Mg, Ni, Zn) metal–organic frameworks have been successfully synthesized at temperatures down to −78 °C in a range of solvent systems and their crystallinity and morphology analyzed by powder X-ray diffraction and scanning electron microscopy. CPO-27-Mg and -Zn could be synthesized at lower temperatures using MeOH–NaOH as the solvent with CPO-27-Zn showing the most crystalline material at −78 °C. CPO-27-Zn afforded the most crystalline samples of all studies in MeOH–TEA. However, in MeOH a non-porous monomeric [Zn(H2dhtp)(H2O)2] complex was formed when no base was present. In THF with base (NaOH, TEA) the reaction produced crystalline MOFs in a controlled and stable manner at low temperatures, whilst the reagents were insoluble in THF at low temperature when no base was present. SEM was used to analyze the morphologies of the products.


 Please wait while we load your content...
Please wait while we load your content...