Copper ion interaction with the RNase catalytic site fragment of the angiogenin protein: an experimental and theoretical investigation†
Abstract
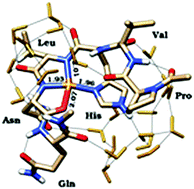
The angiogenin protein (Ang) is a member of the vertebrate-specific secreted ribonucleases and one of the most potent angiogenic factors known. Ang is a normal constituent of human plasma and its concentration increases under some physiological and pathological conditions to promote neovascularization. Ang was originally identified as an angiogenic tumour factor, but its biological activity has been found to extend from inducing angiogenesis to promoting cell survival in different neurodegenerative diseases. Ang exhibits weak ribonucleolytic activity, which is critical for its biological functions. The RNase catalytic sites are two histidine residues, His-13 and His-114, and the lysine Lys-40. Copper is also an essential cofactor in angiogenesis and influences angiogenin's biological properties. The main Cu(II) anchoring site of Ang is His-114, where metal binding inhibits RNase activity of the protein. To reveal the Cu(II) coordination environment in the C-terminal domain of the Ang protein, we report on the characterization, by means of potentiometric, voltammetric, and spectroscopic (CD, UV-Vis and EPR) methods and DFT calculations, of Cu(II) complexes formed with a peptide fragment including the Ang sequence 112–117 (PVHLDQ). Potentiometric titrations indicated that [CuLH−2] is the predominant species at physiological pH. EPR, voltammetric data and DFT calculations are consistent with a CuN3O2 coordination mode in which a distorted square pyramidal arrangement of the peptide was observed with the equatorial positions occupied by the nitrogen atoms of the deprotonated amides of the Asp and Leu residues, the δ-N atom of histidine and the oxygen atom of the aspartic carboxylic group. Moreover, two analogous peptides encompassing the PVHLNQ and LVHLDQ sequences were also characterized by using thermodynamic, spectroscopic and DFT studies to reveal the role they play in Cu(II) complex formation by the carboxylate side chain of the Asp and Pro residues, a known breaking-point in metal coordination.


 Please wait while we load your content...
Please wait while we load your content...