Normal-to-abnormal rearrangement of an N-heterocyclic carbene with a silylene transition metal complex†
Abstract
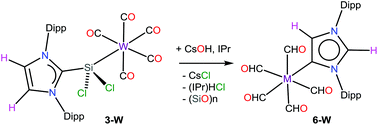
The synthesis and characterization of the N-heterocyclic carbene (NHC) stabilized dichlorosilylene Group 6 metal complexes {(IPr)SiCl2}W(CO)5 (3-W), {(IPr)SiCl2}2Cr(CO)4 (4-Cr), and {(IPr)SiCl2}2W(CO)4 (4-W) (IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene) are reported. Treatment of 3-W with CsOH in the presence of IPr leads to the formation of an abnormal-NHC (aNHC) metal complex (aIPrH)W(CO)5 (6-W) (aIPrH = 1,3-bis(2,6-diisopropylphenyl)imidazol-4-ylidene), unveiling an unprecedented normal-to-abnormal transformation route of an NHC. DFT calculations support the proposed mechanism that involves CsOH mediated deprotonation of the IPr-backbone of 3-W to yield a ditopic carbanionic-NHC (dcNHC) complex 5a-W. Subsequent 1,4-migration of the W(CO)5 moiety and hydrolysis of the unmasked SiCl2 rationalize the formation of 6-W. The desired H2O molecule is generated in the initial step on deprotonation of IPr with CsOH. In contrast to the literature precedents, the calculations indicate that the abnormal complex 6-W is 13.5 kcal mol−1 thermodynamically higher in energy than the normal counterpart (IPr)W(CO)5 (8-W). Interestingly, as the aNHC-compounds reported so far are more stable than their normal counterparts, this finding showcases an opposite trend. Moreover, reaction pathways to the synthesized and related complexes have been investigated by DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...