β-Dicyanovinyl substituted porphyrinogen: synthesis, a reversible sensor for picric acid among explosives and a unique sensor for cyanide and fluoride ions by switching between various porphyrinoid states†
Abstract
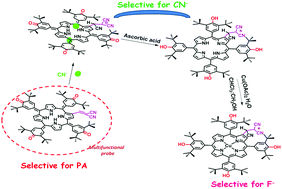
β-Dicyanovinyl substituted porphyrinogen (OxP-MN) was synthesized and utilized as a novel multifunctional sensor for the detection of biologically and environmentally important analytes. OxP-MN (1) acts as a selective and reversible probe for rapid colorimetric detection of picric acid (PA) among other nitroaromatics by switching between two porphyrinoid states. This system displayed a higher β2 value of 1.7 × 108 M−2 and was able to detect PA down to 1.12 ppm (4.99 μM). β-Dicyanovinyl substituted porphyrinogen (OxP-MN) reported here contains a porphyrinogen anion binding site and a dicyanovinyl group as a cyanide-dependent reactive subunit. OxP-MN displayed the first evidence that a β-electron acceptor through a vinyl linker in the case of porphyrinogen results in only an abated shift in the spectrum in contrast to its porphyrin analogues. Porphyrinogen OxP-MN (1) can be switched between a number of porphyrinoid states such as metalloporphodimethene, metalloporphyrin, porphyrinogen, etc. by using CN−, F− and other basic anions. In addition, OxP-MN unveils the unique property of detecting toxic cyanide ions and fluoride ions when “hidden” within a mixture of other anions. Also, OxP-MN behaves as a dual sensor for picric acid and basic anions such as F−, CN−, OAc−, and H2PO4−via the indicator displacement assay under the unrestricted queue.


 Please wait while we load your content...
Please wait while we load your content...