Complexation of tetraalkyl diglycolamides with trivalent f-cations in a room temperature ionic liquid: extraction and spectroscopic investigations†
Abstract
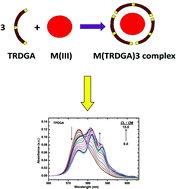
This paper reports the complexation of a series of tetraalkyl diglycolamides (TRDGA) with trivalent f-cations in a room temperature ionic liquid, viz., 3-octyl-1-methylimidazolium bis(trifluoromethanesulfonyl)imide ([C8mim][Tf2N]). The stability constants of TRDGA/Nd3+ complexes (where R = n-pentyl, n-hexyl, n-octyl, n-decyl and 2-ethylhexl) were determined by absorbance spectroscopy. All the DGA ligands formed a 1 : 3 (Nd3+/DGA) complex as the limiting species. Solvent extraction data on Am3+ and Eu3+ were obtained with the TRDGA ligands in [C8mim][Tf2N] to arrive at a correlation between their extraction behaviour and complexation constant. The nature of the complex formed in the single phase titration (in [C8mim][Tf2N]) and those extracted in the [C8mim][Tf2N] medium from the aqueous phase were found to be identical. Fluorescence lifetime data confirmed that, in both single phase titration and biphasic solvent extraction, the complexation proceeded via replacement of water molecules from the primary coordination sphere of the metal ion. The spectroscopic data confirmed the absence of NO3− and Tf2N− ions or H2O in the extracted complexes.


 Please wait while we load your content...
Please wait while we load your content...