Direct synthesis of hydrothermally stable Ge-IWR zeolites†
Abstract
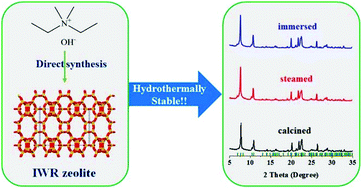
The poor hydrothermal stability of germanosilicate zeolites greatly retards their industrial application. Herein, we present a direct synthesis of Ge-containing IWR zeolites with outstanding hydrothermal stability. A simple quaternary ammonium cation, diethyldimethylammonium, was used as the structure-directing agent for the first time. The structure and chemical composition of the framework was studied by performing Rietveld refinement of XRD data combined with electron microscopy, thermogravimetric and chemical analysis techniques. Al-free and Al-containing IWR zeolites were subjected to high-temperature vapor (973 K, 4 h), water-immersion (room temperature, 12 h) and treatment with 65% HNO3 solution (423 K, 24 h) to test their hydrothermal stability. Materials before and after hydrothermal treatments were characterized by complementary methods (XRD, FTIR, NMR and N2 sorption). The set of experimental data unambiguously proves the high stability of the zeolite framework.


 Please wait while we load your content...
Please wait while we load your content...