The effect of a membrane-mimicking environment on the interactions of Cu2+ with an amyloidogenic fragment of chicken prion protein†
Abstract
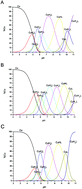
Prion proteins (PrP) from different species have the ability to tightly bind Cu2+ ions. Copper coordination sites are located in the disordered and flexible N-terminal region which contains several His anchoring sites. Among them, two His residues are found in the so called amyloidogenic PrP region which is believed to play a key role in the process leading to oligomer and fibril formation. Both chicken and human amyloidogenic regions have a hydrophobic C-terminal region rich in Ala and Val amino acids. Recent findings revealed that this domain undergoes random coil to α-helix structuring upon interaction with membrane models. This interaction might strongly impact metal binding abilities either in terms of donor sets or affinity. In this study we investigated Cu2+ interaction with an amyloidogenic fragment, chPrP105–140, derived from chicken prion protein (chPrP), in different solution environments. The behavior of the peptide and its metal complexes was analyzed in water and in the presence of negative and positive charged membrane mimicking environments formed by sodium dodecyl sulfate (SDS) and dodecyl trimethyl ammonium chloride (DTAC) micelles. The metal coordination sphere, the metal binding affinity and stoichiometry were evaluated by combining spectroscopic and potentiometric methods. Finally we compare copper(II) interactions with human and chicken amyloidogenic fragments. Our results indicate that the chicken amyloidogenic fragment is a stronger copper ligand than the human amyloidogenic fragment.


 Please wait while we load your content...
Please wait while we load your content...