Non-symmetrical, potentially redox non-innocent imino NHC pyridine ‘pincers’ via a zinc ion template-assisted synthesis†‡
Abstract
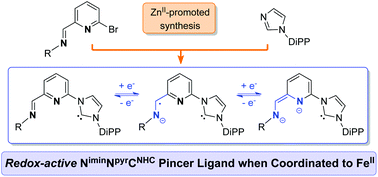
New non-symmetrical, redox-active imino NHC pyridine pincer ligands, 2-(R1-imidazol-2-ylidene)-6-(R2N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)-pyridine (R1 = 2,6-diisopropylphenyl (DiPP), R2 = 2,4,6-trimethylphenyl (Mes), 4B; R1 = R2 = DiPP, 4C), have been accessed by a ZnII-promoted modular synthesis involving the quaternization of R1-imidazole by [Zn(κNimineκNpyridine)(2-(R2N
CH)-pyridine (R1 = 2,6-diisopropylphenyl (DiPP), R2 = 2,4,6-trimethylphenyl (Mes), 4B; R1 = R2 = DiPP, 4C), have been accessed by a ZnII-promoted modular synthesis involving the quaternization of R1-imidazole by [Zn(κNimineκNpyridine)(2-(R2N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)-6-bromo-pyridine)Cl2], followed by ZnII removal and deprotonation of imidazolium pro-ligands. Redox active forms of 4B were implicated in the two complexes obtained by its reaction with FeBr2/KC8; metrical data analysis pointed to the occurrence of radical anionic and dianionic redox states of 4B.
CH)-6-bromo-pyridine)Cl2], followed by ZnII removal and deprotonation of imidazolium pro-ligands. Redox active forms of 4B were implicated in the two complexes obtained by its reaction with FeBr2/KC8; metrical data analysis pointed to the occurrence of radical anionic and dianionic redox states of 4B.


 Please wait while we load your content...
Please wait while we load your content...