Improved reactivity of a cyclic 13/15 compound by increased steric demand†
Abstract
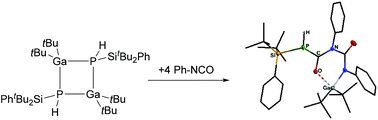
Herein we present the synthesis and characterization of the new four-membered Ga/P cycle [tBu2GaP(H)SitBu2Ph]2, which shows a cis/trans isomerization at ambient temperatures via a ring opening mechanism. The sterically demanding substituents on the phosphorus (-SitBu2Ph) and gallium (tBu) atoms lead to an unexpected reactivity towards bulky NHC ligands (IMes and IDipp). The resulting Lewis base stabilized monomeric 13/15 compounds feature an unusual binding mode of the carbene ligand. The ring opened state also enables a masked flp reactivity, which is shown by a reaction with the polar multiple bond in Ph–NCO.


 Please wait while we load your content...
Please wait while we load your content...