Energetic isomers of 1,2,4,5-tetrazine-bis-1,2,4-triazoles with low toxicity†
Abstract
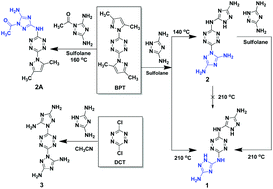
A series of nitrogen-rich “green” Energetic Materials (EMs), some with improved sensitivity, thermostability, and very low toxicity, were synthesized on the basis of 3,5-diamino-1,2,4-triazole (DAT) and 1,2,4,5-tetrazine building blocks. Since DAT contains several nucleophilic reactive sites, obtaining selective reactivity and specific isomeric products with 1,2,4,5-tetrazine precursors is a challenging task. We developed reaction conditions under which specific isomers could be prepared. On evaluating these compounds for their energetic properties, we found that N5,N5′-(1,2,4,5-tetrazine-3,6-diyl)-bis(1H-1,2,4-triazole-3,5-diamine) 1 has very high thermostability (onset of decomposition temperature at 357 °C) and it is insensitive to impact (Im > 98 J), friction (>360 N) and electrostatic discharge (2512 mJ). A detonation velocity (Vod) of 8180 m s−1 was calculated for compound 1 and it was found to have extremely low toxicity in human cells (normal dermal fibroblasts) and in environmental bacteria (Vibrio fischeri). In combination with oxidants, compound 1 can generate 1225 L of gases (per kg of energetic mixture of an oxidant and compound 1), which makes this material a prospective component in solid propellants and a very good candidate for the development of solid state gas generators for clean fire-extinguishing systems and for a broad range of other civil and defense applications that require the use of “green” and insensitive EMs.


 Please wait while we load your content...
Please wait while we load your content...