Exploring the boundaries of direct detection and characterization of labile isomers – a case study of copper(ii)–dipeptide systems†
Abstract
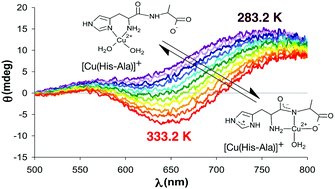
The investigation of the linkage isomers of biologically essential and kinetically labile metal complexes in aqueous solutions poses a challenge, as these microspecies cannot be separately studied. Therefore, derivatives are commonly used to initially determine the stability or spectral characteristics of at least one of the isomers. Here we directly detect the isomers, describe the metal ion coordination sphere, speciation and thermodynamic parameters by a synergistic application of temperature dependent EPR and CD spectroscopic measurements in copper(II)–dipeptide systems including His-Gly and His-Ala ligands. The ΔH = (−23 ± 4) kJ mol−1 value of the standard enthalpy change corresponding to the peptide-type to histamine-type isomerisation equilibrium of the [CuL]+ complex was corroborated by several techniques. The preferential coordination of the side-chains was observed at lower temperatures, whereas, metal-binding of the backbone atoms became favourable upon increasing temperature. This study exemplifies the necessity of using temperature dependent multiple methodologies for a reliable description of similar systems for upstream applications.


 Please wait while we load your content...
Please wait while we load your content...