A small bandgap semiconductor, p-type MnV2O6, active for photocatalytic hydrogen and oxygen production†
Abstract
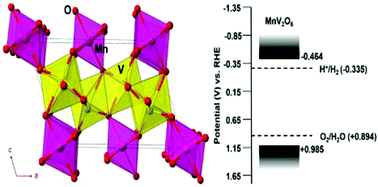
Extensive research has been conducted with the goal to find a single bandgap material that can absorb visible light and efficiently drive the catalysis of water to both hydrogen and oxygen. The p-type MnV2O6 (C2/m, Z = 2, a = 9.289 Å, b = 3.535 Å, and c = 6.763 Å, β = 112.64°), synthesized via solid-state techniques, was investigated for its potential use in the visible-light photocatalysis of water. Mott–Schottky analysis was used to experimentally determine the energetic positions of the valence and conduction bands as +0.985 V and −0.464 V, respectively, at pH 5.68 vs. RHE. These are found to be suitable potentials to drive the reduction and oxidation of water under irradiation. The bandgap transitions, probed using spin-polarized density functional calculations, consist of the excitation of electrons from the half-filled Mn 3d5 orbitals to the empty V 3d0 orbitals. Both hydrogen and oxygen gas were observed as products during suspended-particle photocatalysis experiments under visible-light irradiation. The rate and total moles of gas produced were found to increase with the reaction temperature. As the temperature was raised from 30 °C to 37 °C and 44 °C, the moles of hydrogen produced over 6 hours increased by ∼1.5 and ∼2.5 times. Only oxygen is produced in pure water, showing that methanol is needed to drive hydrogen production.
- This article is part of the themed collection: The Role of Inorganic Materials in Renewable Energy Applications


 Please wait while we load your content...
Please wait while we load your content...