Metal-ion induced ferromagnetic polarization in a mixed-spin system†
Abstract
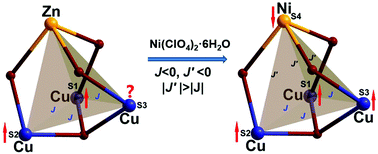
Three new 3d–3d heterometallic complexes, [CuII3MII(hmb)6(OH)]ClO4·H2O (M = Zn (1), Ni (2) and Co (3)) (Hhmb = 2-hydroxy-3-methoxy-benzaldehyde), have been synthesized. Structural analysis reveals that the three complexes are isostructural. Three CuII ions are in a perfect trigonal geometrical frustration and form a tetrahedral frustrated system with the introduced fourth ion ZnII/NiII/CoII. Magnetic studies indicate the antiferromagnetic coupling between metal ions in all 1, 2 and 3. Due to the geometrical frustration, spin-frustrated magnetism is also a typical feature of 1–3 and the magnetostructural correlation is far more complicated than their structures. The detailed magnetic investigation found that the ground state spin of 2 cannot be simply determined by the antiferromagnetically linear arrangement of spins, because the strong antiferromagnetic coupling between CuII and NiII ions quenches the spin frustration and ferromagnetically polarizes the spins of the [CuII3] unit. Contrarily, the antiferromagnetic coupling between CuII and CoII ions is not strong enough in 3, so there is no similar behaviour compared to 2.


 Please wait while we load your content...
Please wait while we load your content...