Tuning the copper(ii) coordination properties of cyclam by subtle chemical modifications†
Abstract
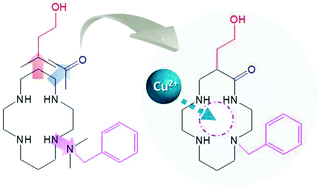
The acid–base and copper(II) coordination properties of three previously described cyclam derivatives are reported. Potentiometry, mass spectrometry, UV-vis absorption spectroscopy, electrochemistry and theoretical calculations were combined to investigate the protonation and binding properties of Bn-cyclam-EtOH (L1), oxo-cyclam-EtOH (L2) and oxo-Bn-cyclam-EtOH (L3). These three cyclams are C-functionalized by a hydroxyethyl pendant arm and display either one N-benzyl group and/or an amide replacing one macrocyclic secondary amine. The N-benzylic substitution has a significant effect of lowering the basicity of the corresponding protonation sites, while the presence of the amide function lowers the first protonation constants of the ligands. Regardless of the system considered, ESI mass spectrometry showed that only monocupric chelates are formed. Compared to the literature data, the stability constants measured by potentiometry (pCu L1 = 14.67; pCu L2 = 16.95; pCu L3 = 15.28) showed that: (i) the C-appended group has a negligible influence on Cu2+ complexation, (ii) N-benzylation decreases the cupric complex stability, and (iii) the “oxo” function significantly increases the stability of the Cu2+ complex. Furthermore, UV-vis absorption versus pH measurements are in excellent agreement with the potentiometric titrations and show an equal involvement of the four nitrogen atoms in L1 and the strong binding properties of L2 and L3 related to the deprotonation of the carboxamide. The electrochemistry parameters determined by cyclic voltammetry showed the predominance of the [CuL1]2+, [CuL2-H]+ and [CuL3-H]+ species but also the irreversibility of the three Cu2+/Cu+ systems. Finally, density functional theory (DFT) and multiconfigurational CASSCF/NEVPT2 calculations confirmed that the protonation of the cupric complexes occurs at the oxygen atom of the amide group of the “oxo” ligands, which is in agreement with the experimental data.


 Please wait while we load your content...
Please wait while we load your content...