Spectroscopic evidence for cofactor–substrate interaction in the radical-SAM enzyme TYW1†
Abstract
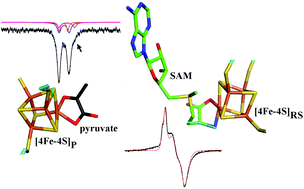
TYW1 is a metalloenzyme involved in the modifications of guanosine 37 of Phe-tRNA of Eukaryota and Archaea. It catalyzes the second step of Wybutosine biosynthesis, which consists of the formation of the tricyclic compound imG-14 from m1G using pyruvate and SAM (S-adenosyl-methionine) as co-substrates. Two [4Fe–4S] clusters are needed in the catalytic process. One effects the reductive binding of SAM, which initiates the radical reaction that inserts a C–C moiety into m1G. The other [4Fe–4S] cluster binds the pyruvate molecule that provides the C–C motif. Using a combination of EPR and Mössbauer spectroscopy, we have been able to probe the binding of both cofactors to the FeS clusters. The results highlight an interaction between pyruvate and SAM, indicating that they bind in close vicinity inside the catalytic pocket. They also indicate a chelating binding mode of pyruvate to the accessible Fe site of the corresponding FeS cluster. This binding mode has been used to construct a docking model of holoTYW1 with pyruvate and SAM, which is consistent with the spectroscopic findings.
- This article is part of the themed collection: Frontiers in Spectroscopic Techniques in Inorganic Chemistry


 Please wait while we load your content...
Please wait while we load your content...