Synthesis and characterization of a family of thioether-dithiolate-bridged heteronuclear iron complexes†
Abstract
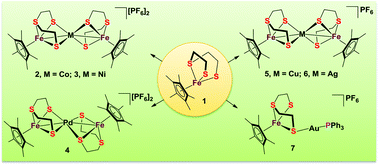
The thioether-dithiolate-bridged heterotrinuclear complexes [Cp*Fe(μ-1k3SSS′:2k2SS-tpdt)M(μ-2k2SS:3k3SSS′-tpdt)FeCp*][PF6]2 (Cp* = η5-C5Me5; tpdt = S(CH2CH2S)2; 2, M = Co; 3, M = Ni; 4, M = Pd) have been prepared by a reaction of [Cp*Fe(η3-tpdt)] (1) with complexes CoCl2, NiCl2(PPh3)2, and PdCl2(PPh3)2, respectively. Similarly, treatment of complex 1 with CuCl(PPh3) or AgPF6 afforded two heterotrinuclear complexes, [Cp*Fe(μ-1k3SSS′:2k2SS-tpdt)M(μ-2k2SS:3k3SSS′-tpdt)FeCp*][PF6] (5, M = Cu; 6, M = Ag), while reaction of 1 with the complex AuCl(PPh3) gave a heterobinuclear complex, [Cp*Fe(μ-1k3SSS′:2k1S-tpdt)Au(PPh3)][PF6] (7). These complexes have been spectroscopically and crystallographically characterized. An X-ray diffraction analysis showed that complexes 2, 3, 5, and 6 feature a heterometal center binding four sulfur atoms of two tpdt ligands with a cis orientation. However, in the Pd-containing complex 4, two tpdt ligands are arranged in a trans configuration. The μeff data and EPR results indicate that complexes 2, 4, 5, 6, and 7 are paramagnetic and only complex 3 is diamagnetic. Electrochemical experiments on these heteronuclear clusters were performed at room temperature. Discrepancy of the redox couples in the CV plots of these complexes indicates different one-electron transfer processes.


 Please wait while we load your content...
Please wait while we load your content...