Pyrazolates advance cerium chemistry: a CeIII/CeIV redox equilibrium with benzoquinone†
Abstract
Two stable cerium(IV) 3,5-dialkylpyrazolate complexes are presented, namely dimeric [Ce(Me2pz)4]2 (Me2pz = 3,5-dimethylpyrazolate) and monomeric Ce(tBu2pz)4 (tBu2pz = 3,5-di-tert-butylpyrazolate) along with their trivalent counterparts [Ce(Me2pz)3] and [Ce(tBu2pz)3]2. All complexes were obtained from protonolysis reactions employing the silylamide precursors Ce[N(SiHMe2)2]4 and Ce[N(SiMe3)2]3. Treatment of homoleptic CeIV and CeIII Me2pz complexes with 1,4-hydroquinone (H2hq) or 1,4-benzoquinone (bq), respectively, ultimately gave the same trimetallic CeIII species via a cerium redox equilibrium. The CeIII complex Ce3(Me2pz)5(pchd)2(L) (pchd = 1,4-bis(3,5-dimethylpyrazol-1-yl)cyclohex-2,5-diene-1,4-diolato; L = Me2pzH or (thf)2) results from a di-1,4-pyrazolyl attack on pre-coordinated bq. The reduction of bq by [Ce(Me2pz)3(thf)]2, and re-oxidation by the resulting CeIV species was supported by UV-vis spectroscopic investigations. Comparisons with the redox-innocent complexes [Ln(Me2pz)3(thf)]2 (Ln = La and Pr) revealed far less selective reactions with bq, giving hexametallic and octametallic rare-earth metal side products containing 2-Me2pz substituted hq ligands.
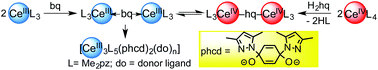


 Please wait while we load your content...
Please wait while we load your content...