Speciation of indium(iii) chloro complexes in the solvent extraction process from chloride aqueous solutions to ionic liquids†
Abstract
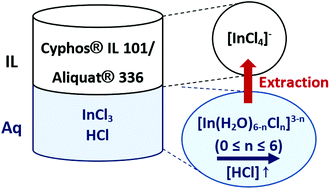
Most metal extraction studies focus on the kinetics, the maximum loading and the extraction equilibrium, while structural information on the extracted complexes has been limited. This paper concerns the nature of the indium(III) chloride complexes, present in the organic and aqueous phase during the solvent extraction of indium(III) from an aqueous HCl solution by undiluted ionic liquids Cyphos® IL 101 and Aliquat® 336. In an aqueous HCl solution (0–12 M), indium(III) exists as octahedral mixed complexes, [In(H2O)6−nCln]3−n (0 ≤ n ≤ 6). EXAFS and 115In NMR were used to characterize these species. The stoichiometric composition of the extracted complexes, which is estimated from viscosity and maximum loading studies and confirmed by EXAFS, is unaffected by the HCl concentration in the aqueous phase. Indium(III) is present in the ionic liquid phase as the tetrahedral [InCl4]− complex. Based on the speciation results an extraction mechanism is proposed.
- This article is part of the themed collection: Inorganic chemistry approaches to saving critical elements: Recovery, Reuse and Recycling


 Please wait while we load your content...
Please wait while we load your content...