Comparisons of MN2S2vs. bipyridine as redox-active ligands to manganese and rhenium in (L–L)M′(CO)3Cl complexes†
Abstract
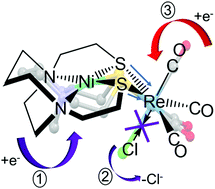
The bipyridine ligand is renowned as a photo- and redox-active ligand in catalysis; the latter has been particularly explored in the complex Re(bipy)(CO)3Cl for CO2 reduction. We ask whether a bidentate, redox-active MN2S2 metallodithiolate ligand in heterobimetallic complexes of Mn and Re might similarly serve as a receptor and conduit of electrons. In order to assess the electrochemical features of such designed bimetallics, a series of complexes featuring redox active MN2S2 metallodithiolates, with M = Ni2+, {Fe(NO)}2+, and {Co(NO)}2+, bound to M′(CO)3X, where M′ = Mn and Re, were synthesized and characterized using IR and EPR spectroscopies, X-ray diffraction, cyclic voltammetry, and density functional theory (DFT) computations. Butterfly type structures resulted from binding of the convergent lone pairs of the cis-sulfur atoms to the M′(CO)3X unit. Bond distances and angles are similar across the M′ metal series regardless of the ligand attached. Electrochemical characterizations of [MN2S2·Re(CO)3Cl] showed the redox potential of the Re is significantly altered by the identity of the metal in the N2S2 pocket. DFT calculations proved useful to identify the roles played by the MN2S2 ligands, upon reduction of the bimetallics, in altering the lability of the Re–Cl bond and the ensuing effect on the reduction of ReI to Re0.


 Please wait while we load your content...
Please wait while we load your content...