On the Lewis acidic character of bis(salicylaldiminato)zinc(ii) Schiff-base complexes: a computational and experimental investigation on a series of compounds varying the bridging diimine†
Abstract
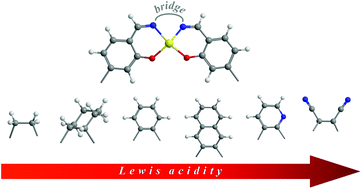
This contribution explores the effect of the 1,2-diimine bridge upon the Lewis acidic character of a series of bis(salicylaldiminato)zinc(II), ZnL, Schiff-base complexes. The structure of the monomeric and dimeric ZnL complexes, and of the 1 : 1 adducts with pyridine, ZnL·py, is fully optimized by means of DFT calculations. The Gibbs free energy for the dimerization of ZnL complexes and for the formation of ZnL·py adducts is evaluated by accurate composite calculations. It accounts for their spontaneous dimerization and for the greater stability of the ZnL·py adducts with respect to the dimers. Calculated binding constants for the formation ZnL·py adducts are in excellent agreement with experimentally derived values, thus allowing establishing a relative Lewis acidity scale within this series. While the complex derived from the non-conjugated ethylenediamine reveals the lowest Lewis acidity, the complex derived from the diaminomaleonitrile represents the stronger Lewis acidic species. These findings are in good agreement with the greater catalytic activity observed for ZnL Schiff-base complexes derived from conjugated 1,2-diamines in comparison to the non-conjugated analogues. Both in ZnL dimers as well as in ZnL·py adducts the geometry of the coordination sphere seems to be a relevant feature to assess their relative stability. Thus, while the quasi-planarity of ZnL monomers of the conjugated diimines is an unfavourable feature in the dimerization process, it represents an important aspect in stabilizing ZnL·py adducts in a nearly perfect square-pyramidal coordination. These features are relevant for the sensing and catalytic properties of these complexes.


 Please wait while we load your content...
Please wait while we load your content...