Flexible organofunctional aerogels†
Abstract
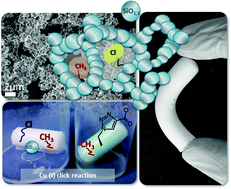
Flexible inorganic–organic silica aerogels based on methyltrimethoxysilane (MTMS, CH3Si(OCH3)3) can overcome the drawbacks of conventional silica aerogels by introducing high mechanical strength, elastic recovery and hydrophobicity to monolithic materials. In this work, MTMS is co-condensed with organofunctional alkoxysilanes RSi(OMe)3 (R = vinyl, chloropropyl, mercaptopropyl, methacryloxypropyl, etc.) yielding aerogels that are not only flexible but also contain reactive functional groups. Sol–gel parameters, such as the MTMS/RSi(OMe)3 ratio, have been systematically investigated in terms of gelation behavior, complete/incomplete incorporation of the functional organic groups (confirmed by FTIR-ATR and Raman spectroscopy) and flexibility of the resulting gel. Sterically more demanding functional moieties lead to macroscopic phase separation; however, this problem was overcome by the employment of surfactants. Functional aerogels dried by supercritical extraction with carbon dioxide showed promising results in uniaxial compression tests and had an elastic recovery up to 60%. Furthermore, the accessibility of the functional groups was demonstrated by simple reactions, e.g. conversion of the chloro into azido groups via a nucleophilic substitution reaction with NaN3 followed by click reactions.
- This article is part of the themed collection: Silicon Chemistry: Discoveries and Advances


 Please wait while we load your content...
Please wait while we load your content...